Log in to start sending quotation requests for any product.
Don't have an account? Sign Up Here
Home Oxides

Oxides are chemical compounds formed when oxygen combines with other elements. They can be classified into acidic, basic, or amphoteric oxides based on their chemical properties. Acidic oxides react with water to form acidic solutions, while basic oxides form basic solutions. Amphoteric oxides can exhibit both acidic and basic properties depending on the conditions. Common examples include carbon dioxide (CO2), which can form an acidic solution when dissolved in water, and calcium oxide (CaO), known as quicklime, which reacts with water to produce a basic solution. Oxides have a broad range of applications, from industrial uses like in metal smelting to everyday applications like in building materials, as well as playing essential roles in biological and environmental systems.
Explore our network of country and industry based websites to access localized information, product offerings, and business services across our group.
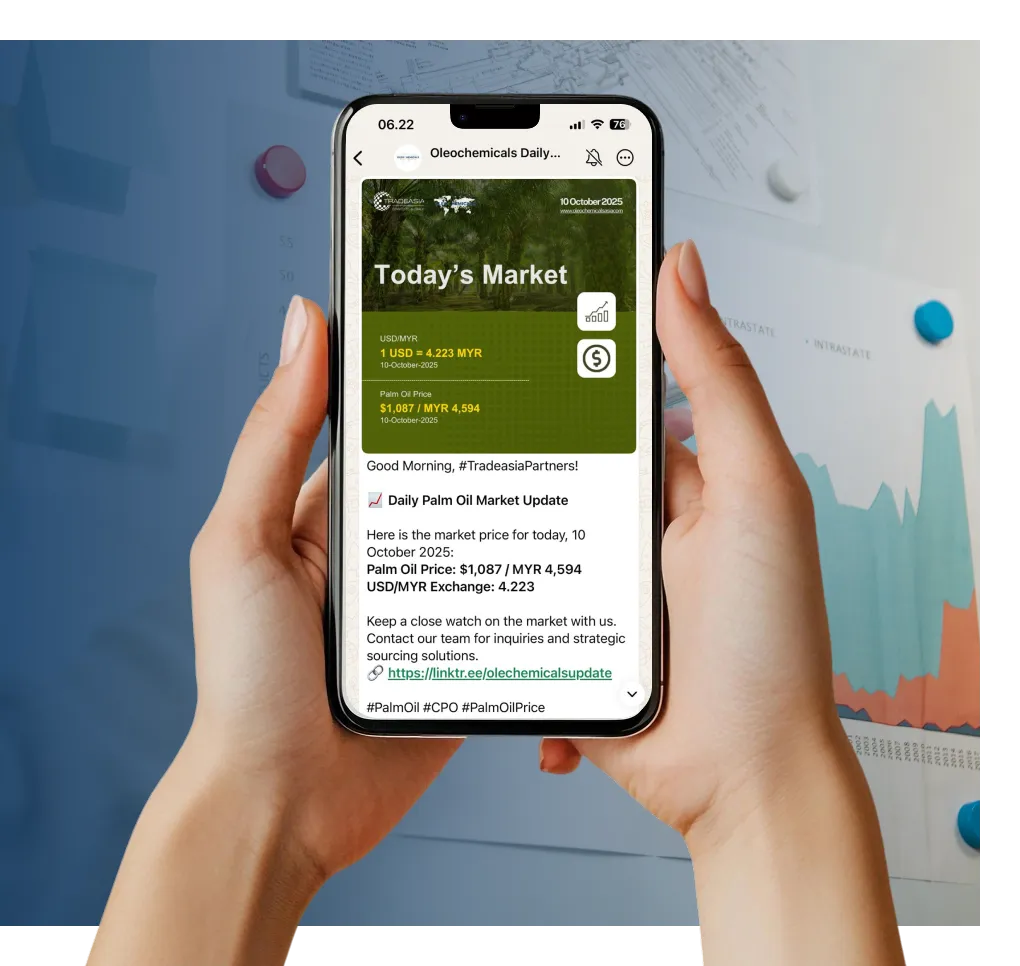
Access reliable chemical market information through our update channels.
Real-time Updates
Daily Updates


